What are Acids, Bases, and Salts?
Many acids and bases occur naturally in nature, such as citric acid in fruits like orange, lemon, etc, tartaric acid in tamarind, malic acid in apples, and lactic acid in milk and milk products, hydrochloric acid in gastric juices.
Similarly, many bases are found such as lime water. We use many of these acids in our day-to-day life, such as vinegar or acetic acid in the kitchen, boric acid for laundry, baking soda for the purpose of cooking, washing soda for cleaning, etc.
Many of the acids that we do not consume in the household are used in the laboratories and industries, which include an acid such as HCl, H2SO4 etc, and bases such as NaOH, KOH etc. When these acids and bases are mixed in the right proportions, the neutralization reaction thus results in the formation of salt and water. Some naturally occurring salts found in nature include NaCl and KCl etc in seawater and natural rock deposits. In this section, we will read more about acid, base, and salt and their properties.
Definitions
- Acid:- An acid is defined as a substance whose water solution tastes sour, turns blue litmus red, and neutralizes bases.
- Base:- A substance is called base if its aqueous solution tastes bitter, turns red litmus blue, or neutralizes acids.
- Salt:- Salt is a neutral substance whose aqueous solution does not affect litmus.
Acids
The term acid is derived from a Latin word ‘acidus’ or ‘acere’, which means sour. The most common characteristic is their sour taste. An acid is a substance that renders ionizable hydronium ion (H3O+) in its aqueous solution. It turns blue litmus paper red. These dissociate in their aqueous solution to form their constituent ions, as given by the following examples.
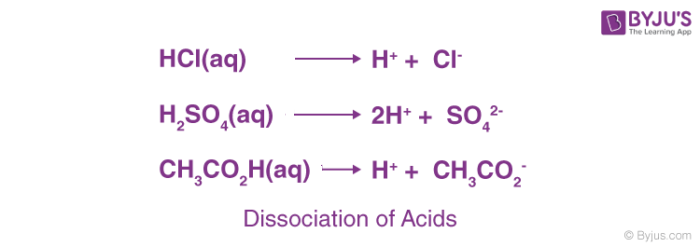

Based on their occurrence, they are divided into two types- Natural and mineral acids.
Natural Acids: These are obtained from natural sources, such as fruits and animal products. For e.g. lactic, citric, and tartaric acid etc.
Mineral Acids: Mineral acids are acids prepared from minerals. Examples are Hydrochloric acid (HCl), Sulphuric Acid (H2SO4), and nitric acid (HNO3), etc.
Bases
The most common characteristic of bases is their bitter taste and soapy feel. A base is a substance that renders hydroxyl ion(OH–) in their aqueous solution. Bases turn the colour of red litmus paper to blue.

The bases dissociate in their aqueous solution to form their constituent ions, given in the following examples.
Salts
Salt is an ionic compound that results from the neutralization reaction of acids and bases. Salts are constituted of positively charged ions, known as cations, and negatively charged ions, known as anions, which can either be organic or inorganic in nature. These ions are present in a relative amount, thus rendering the nature of the salt neutral.

The formation of salt can be seen from the chemical reactions shown in the equations below.
A salt is formed when hydrogen ions are replaced by a metal or an ammonium ion in an acid. A base is a material that reacts with an acid to produce just water and salt. When an acid reacts with a base, it produces a salt.
Bases are commonly found in household cleansers that are used to remove oil from windows and floors, as well as soaps, toothpaste, egg whites, dishwashing liquids, and household ammonia.
Introduction to Acids, Bases and Salts
A substance that tastes sour in water, turns blue litmus red, and neutralises the bases is known as an acid. If a substance’s aqueous solution tastes bitter, turns red litmus blue, or neutralises acids, it’s called a base. Salt is a neutral material that has no effect on litmus in an aqueous solution.
Classification of Matter
On the basis of
a) Composition – elements, compounds and mixtures
b) State – solids, liquids and gases
c) Solubility – suspensions, colloids and solutions
Types of mixtures – homogeneous and heterogeneous
Types of compounds – covalent and ionic
What Is an Acid and a Base?
Ionisable and Non-Ionisable Compounds
An ionisable compound, when dissolved in water or in its molten state, dissociates into ions almost entirely. Examples: NaCl, HCl, KOH, etc.
A non-ionisable compound does not dissociate into ions when dissolved in water or in its molten state. Examples: glucose, acetone, etc.
Acids and Bases
An acid is any hydrogen-containing substance that is capable of donating a proton (hydrogen ion) to another substance. A base is a molecule or ion able to accept a hydrogen ion from an acid. Acidic substances are usually identified by their sour taste.
Arrhenius’ Theory of Acids and Bases
Arrhenius acid – when dissolved in water, dissociates to give H+ (aq) or H3O+ ion.
Arrhenius base – when dissolved in water, dissociates to give OH− ion.
Examples
Acids
- Hydrochloric acid (HCl)
- Sulphuric acid (H2SO4)
- Nitric acid (HNO3)
Bases
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Calcium hydroxide (Ca(OH)2)
Bronsted Lowry Theory
A Bronsted acid is an H+ (aq) ion donor.
A Bronsted base is an H+ (aq) ion acceptor.
Example
In the reaction: HCl (aq) + NH3 (aq) → NH+4(aq) + Cl− (aq)
HCl – Bronsted acid and Cl− : its conjugate acid
NH3 – Bronsted base and NH+4 : its conjugate acid
Physical Test
Given are two possible physical tests to identify an acid or a base.
a. Taste
An acid tastes sour, whereas a base tastes bitter.
The method of taste is not advised, as an acid or a base could be contaminated or corrosive.
Example: The flavours of curd, lemon juice, orange juice, and vinegar are all sour. Because they contain acids, these compounds have a sour flavour. Baking soda has a sour flavour. It’s an example of a foundation.
b. Effect on Indicators by Acids and Bases
An indicator is a chemical substance which shows a change in its physical properties, mainly colour or odour, when brought in contact with an acid or a base.
Below mentioned are commonly used indicators and the different colours they exhibit:
a) Litmus
In a neutral solution – purple
In an acidic solution – red
In a basic solution – blue
Litmus is also available as strips of paper in two variants – red litmus and blue litmus.
An acid turns a moist blue litmus paper to red.
A base turns a moist red litmus paper to blue.
b) Methyl orange
In a neutral solution – orange
In an acidic solution – red
In a basic solution – yellow
c) Phenolphthalein
In a neutral solution – colourless
In an acidic solution – remains colourless
In a basic solution – pink
Acid-Base Reactions
A neutralisation reaction occurs when an acid reacts with a base. Salt and water are the end products of this reaction. An acid–base neutralisation reaction is formulated as a double-replacement reaction in this standard approach.
Reactions of Acids and Bases
a) Reaction of acids and bases with metals
Acids, in general, react with metals to produce salt and hydrogen gas. Bases, in general, do not react with metals and do not produce hydrogen gas.
Acid + active metal → salt + hydrogen + heat
2HCl + Mg → MgCl2 + H2 (↑)
Hydrochloric acid + Magnesium → Magnesium chloride + Hydrogen
Base + metal → salt + hydrogen + heat
2NaOH + Zn → Na2ZnO2 + H2 (↑)
Sodium hydroxide + Zinc → Sodium zincate + Hydrogen
A more reactive metal displaces the less reactive metal from its base.
2Na + Mg (OH) 2 → 2NaOH + Mg
Sodium + Magnesium hydroxide → Sodium hydroxide + Magnesium
b) Reaction of acids with metal carbonates and bicarbonates
Acids produce carbon dioxide, as well as metal salts and water, when they react with metal carbonates or metal bicarbonates. Sodium chloride, carbon dioxide, and water are formed when sodium carbonate interacts with hydrochloric acid. Allowing carbon dioxide gas to travel through lime water turns it milky.
Acid + metal carbonate or bicarbonate → salt + water + carbon dioxide.
2HCl + CaCO3 → CaCl2 + H2O + CO2
H2SO4 + Mg (HCO3)2 → MgSO4 + 2H2O + 2CO2
Effervescence indicates the liberation of CO2 gas.
c) Reaction of Acid with Base
1. Reaction of metal oxides and hydroxides with acids
Metal oxides or metal hydroxides are basic in nature.
Acid + base → salt + water + heat
H2SO4 + MgO → MgSO4 + H2O
2HCl + Mg (OH) 2 → MgCl2 + 2H2O
2. Reaction of non-metal oxides with bases
Non-metal oxides are acidic in nature
Base + Nonmetal oxide → salt + water + heat
2NaOH + CO2→ Na2CO3 + H2O
3. Reaction of acids and base
A very common acid is hydrochloric acid. The reaction between strong acid, says hydrochloric acid and strong base say sodium hydroxide, forms salt and water. The complete chemical equation is shown below.
HCl (strong acid) + NaOH (strong base) → NaCl (salt) + H2O (water)
Water
Acids and Bases in Water
When added to water, acids and bases dissociate into their respective ions and help in conducting electricity.
Difference between a Base and an Alkali
Base:
- Bases undergo a neutralisation reaction with acids.
- They are comprised of metal oxides, metal hydroxides, metal carbonates and metal bicarbonates.
- Most of them are insoluble in water.
Alkali:
- An alkali is an aqueous solution of a base, (mainly metallic hydroxides).
- It dissolves in water and dissociates to give OH− ion.
- All alkalis are bases, but not all bases are alkalis.
Hydronium Ion
Hydronium ion is formed when a hydrogen ion accepts a lone pair of electrons from the oxygen atom of a water molecule, forming a coordinate covalent bond.

Dilution
Dilution is the process of reducing the concentration of a solution by adding more solvent (usually water) to it.
It is a highly exothermic process.
To dilute acid, the acid must be added to water and not the other way round.
Strength of Acids and Bases
Strong acid or base: When all molecules of a given amount of an acid or a base dissociate completely in water to furnish their respective ions, H+(aq) for acid and OH−(aq) for base).
Weak acid or base: When only a few of the molecules of a given amount of an acid or a base dissociate in water to furnish their respective ions, H+(aq) for acid and OH−(aq) for base).
Dilute acid: contains less number of H+(aq) ions per unit volume.
Concentrated acid: contains more number of H+(aq) ions per unit volume.

Universal Indicator
A universal indicator has a pH range from 0 to 14 that indicates the acidity or alkalinity of a solution.
A neutral solution has pH=7
pH
pH=−log10[H+]
In pure water, [H+]=[OH−]=10−7 mol/L. Hence, the pH of pure water is 7.
The pH scale ranges from 0 to 14.
If pH < 7 → acidic solution
If pH > 7→ basic solution


pH scale
Importance of pH in Everyday Life
1. pH sensitivity of plants and animals
Plants and animals are sensitive to pH. Crucial life processes such as digestion of food, functions of enzymes and hormones happen at a certain pH value.
2. pH of a soil
The pH of a soil optimal for the growth of plants or crops is 6.5 to 7.0.
3. pH in the digestive system
The process of digestion happens at a specific pH in our stomach which is 1.5 to 4.
The pH of the interaction of enzymes, while food is being digested, is influenced by HCl in our stomach.
4. pH in tooth decay
Tooth decay happens when the teeth are exposed to an acidic environment of pH 5.5 and below.
5. pH of self-defence by animals and plants
Acidic substances are used by animals and plants as a self-defence mechanism. For example, bee and plants like nettle secrete a highly acidic substance for self-defence. These secreted acidic substances have a specific pH.
Manufacture of Acids and Bases
Manufacture of acids and bases
a) Nonmetal oxide + water → acid
SO2(g) + H2O(l) → H2SO3(aq)
SO3(g) + H2O(l) → H2SO4(aq)
4NO2(g) + 2H2O(l) + O2(g) → 4HNO3(aq)
Non-metal oxides are thus referred to as acid anhydrides.
b) Hydrogen + halogen → acid
H2(g) + Cl2(g) → 2HCl(g)
HCl(g) + H2O(l) → HCl(aq)
c) Metallic salt + conc. sulphuric acid → salt + more volatile acid
2NaCl(aq) + H2SO4(aq) → Na2SO4(aq) + 2HCl(aq)
2KNO3(aq) + H2SO4(aq) → K2SO4(aq) + 2HNO3(aq)
d) Metal + oxygen → metallic oxide (base)
4Na(s) + O2(g) → 2Na2O(s)
2Mg(s) + O2(g) → 2MgO(s)
e) Metal + water → base or alkali + hydrogen
Zn(s) + H2O(steam) → ZnO(s)+ H2(g)
f) Few metallic oxides + water → alkali
Na2O(s) + H2O(l) → 2NaOH(aq)
g) Ammonia + water → ammonium hydroxide
NH3(g) + H2O(l) → NH4OH(aq)
Salts
Salt is a combination of an anion of an acid and a cation of a base.
Examples – KCl, NaNO3 , CaSO4, etc.
Salts are usually prepared by the neutralisation reaction of an acid and a base.
Common Salt
Sodium Chloride (NaCl) is referred to as common salt because it’s used all over the world for cooking.
Family of Salts
Salts having the same cation or anion belong to the same family. For example, NaCl, KCl, LiCl.
pH of Salts
A salt of a strong acid and a strong base will be neutral in nature. pH = 7 (approx.).
A salt of a weak acid and a strong base will be basic in nature. pH > 7.
A salt of a strong acid and a weak base will be acidic in nature. pH < 7.
The pH of a salt of a weak acid and a weak base is determined by conducting a pH test.
Chemicals from Common Salt
Sodium chloride is a common salt. NaCl is its molecular formula. The fundamental element in our meals is sodium chloride. It is used in our food as a flavour enhancer as well as a preservative. From common salt, we may make the following four compounds.
- Sodium hydroxide or lye or caustic soda
- Baking soda or sodium hydrogen carbonate, or sodium bicarbonate
- Washing soda or sodium carbonate decahydrate
- Bleaching powder or calcium hypochlorite
Preparation of Sodium Hydroxide
The strong base sodium hydroxide is a common and useful one. Preparing a solution of sodium hydroxide (NaOH) in water requires extra caution because the exothermic reaction releases a lot of heat. It’s possible that the solution will spatter or boil. Here’s how to manufacture a sodium hydroxide solution safely, as well as recipes for a variety of NaOH strengths.
Chemical formula – NaOH
Also known as – caustic soda

Preparation (Chlor-alkali process):
Electrolysis of brine (solution of common salt, NaCl) is carried out.
At anode: Cl2 is released
At cathode: H2 is released
Sodium hydroxide remains in the solution.
Bleaching Powder
Bleaching powder is soluble in water and is used as a bleaching agent in textile industries. It is also used as an oxidizing agent and a disinfectant in many industries. It should also be noted that bleaching powder is synthesized by the reacting chlorine gas on dry slaked lime i.e. Ca(OH)2.
Chemical formula – Ca(OCl)Cl or CaOCl2
Preparation – Ca(OH)2(aq)+Cl2(g)→CaOCl2(aq)+H2O(l)
On interaction with water – bleaching powder releases chlorine which is responsible for bleaching action.
Uses of Bleaching Powder
- It is used for bleaching dirty clothes in the laundry, as a bleaching agent for cotton and linen in the textile industry.
- It is a strong oxidizing agent, hence used as an oxidizer in many industries.
- It is used as a disinfectant which is used for disinfecting water to make potable water.
Baking Soda
Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3 and the IUPAC designation sodium hydrogen carbonate. A sodium cation (Na+) and a bicarbonate anion (HCO3) combine to form this salt. Sodium bicarbonate is a white, crystalline substance that is commonly found as a fine powder. It tastes slightly salty and alkaline, like washing soda (sodium carbonate).
Chemical name – Sodium hydrogen carbonate
Chemical formula – NaHCO3
Preparation (Solvay process):
a. Limestone is heated: CaCO3→CaO+CO2
b. CO2 is passed through a concentrated solution of sodium chloride and ammonia:
NaCl(aq)+NH3(g)+CO2(g)+H2O(l)→NaHCO3(aq)+NH4Cl(aq)
Uses:
- Reduces the acidity in the stomach
- Acts as an antacid which is used to treat stomach upset and indigestion
- Used in the process of washing as a water softener
Washing Soda
Chemical name – Sodium hydrogen carbonate
Chemical formula – NaHCO3
Preparation (Solvay process) –
a. Limestone is heated: CaCO3 → CaO + CO2
b. CO2 is passed through a concentrated solution of sodium chloride and ammonia:
NaCl(aq) + NH3(g) + CO2(g) + H2O(l) → NaHCO3(aq) + NH4Cl(aq)
Uses
1. In the glass, soap and paper industries
2. Softening of water
3. Domestic cleaner
Crystals of Salts
Certain salts form crystals by combining with a definite proportion of water. The water that combines with the salt is called water of crystallisation.
The process by which a solid form, in which the atoms or molecules are strongly arranged into a structure known as a crystal, is known as crystallisation. Precipitation from a solution, freezing, and, more rarely, direct deposition from a gas are some of the ways crystals form.
Example:
Table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes are examples of common materials that form crystals. Many gemstones, such as quartz and diamond, are crystals.
Plaster of Paris
Plaster of Paris is a widely used chemical compound that is extensively used in sculpting materials and gauze bandages. Plaster of Paris is a white powdery chemical compound that is hydrated calcium sulphate that is usually obtained by calcining gypsum. While we have seen many applications of this material in our everyday lives, if we try to understand its chemistry, we will find that it is a white powdery chemical compound that is hydrated calcium sulphate that is usually obtained by calcining gypsum. To put it another way, Plaster of Paris is often manufactured of heated gypsum at a high temperature.
Gypsum plaster is another name for the plaster of Paris. Plaster of Paris is expressed as CaSO4. ½ H2O in the chemical formula.
Gypsum, CaSO4.2H2O (s) on heating at 100°C (373K) gives CaSO4. ½ H2O and 3/2 H2O
CaSO4. ½ H2O is plaster of Paris.
CaSO4. ½ H2O means two formula units of CaSO4 share one molecule of water.
Uses – cast for healing fractures.







0 Comments