Atomic Symbol
Atom of an element is represented in the form of an atomic symbol.
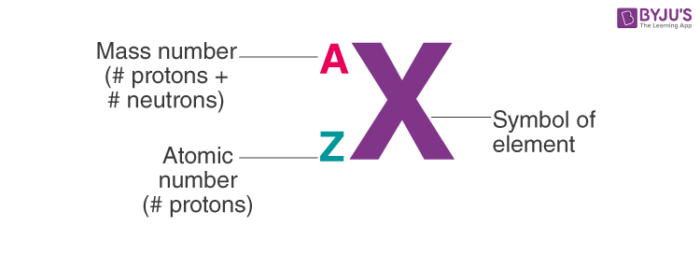

Atomic Number (z)
The number of protons present in the nucleus of an atom is equal to its atomic number (Z).
Thus Atomic number (Z) = number of protons in the nucleus of an atom
Example Atomic number of Carbon(C) = 6 = number of protons
Atomic Mass (A)
Atomic mass is equal to the sum of protons and neutrons present in the nucleus of the atom.
Thus Atomic mass = number of protons + number of neutrons
For Example Atomic mass of carbon = 12






0 Comments