Atomic Valency
Molecules and Atomicity
A molecule is defined as the smallest unit of a compound that contains the chemical properties of the compound.
- The atomicity of an element is the number of atoms in one molecule of the element.
- For e.g., Hydrogen, nitrogen, oxygen, chlorine, iodine, and bromine all have two atoms in each of their molecules. So, the atomicity of hydrogen, nitrogen, oxygen, chlorine, iodine, and bromine is two each.
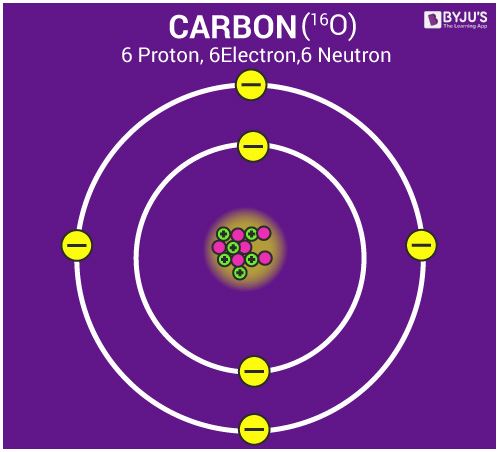
Structure of an Atom
- Atom is made of three particles; electron, proton and neutron.
- The centre of the atom is called the nucleus. The nucleus of an atom contains the whole mass of an atom.
- Electrons in an atom are arranged in shells/orbitals.
Valency
Valence electrons are those electrons which are present in the outermost orbit of the atom.
- The capacity of an atom to lose, gain or share valence electrons in order to complete its octet determines the valency of the atom.






0 Comments