What is a Chemical Reaction?
A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance.
Chemical reactions are all around us, from the metabolism of food in our body to how the light we get from the sun is the result of chemical reactions. Before beginning with chemical reactions, it is important to know about physical and chemical changes.

A burning candle is the best example of physical and chemical change. Take a candle and light it. As time passes, we can observe that the candle changes to wax. If you cover the candle with a jar, it will extinguish.
In the demonstration, burning of the candle is a chemical change while conversion of the candle to wax is a physical change. In a physical change, there is basically a change of state of the substance but in the case of a chemical change mostly a new substance is formed in which either energy is given off or absorbed. Thus, we can conclude that chemical changes are accompanied by certain physical changes.
Basic Concepts of Chemical Reactions
- A Chemical Reaction is a process that occurs when two or more molecules interact to form a new product(s).
- Compounds that interact to produce new compounds are called reactants whereas the newly formed compounds are called products.
- Chemical reactions play an integral role in different industries, customs and even in our daily life. They are continuously happening in our general surroundings; for example, rusting of iron, pottery, fermentation of wine and so on.
- In a chemical reaction, a chemical change must occur which is generally observed with physical changes like precipitation, heat production, colour change etc.
- A reaction can take place between two atoms or ions or molecules, and they form a new bond and no atom is destroyed or created but a new product is formed from reactants.
- The rate of reaction depends on and is affected by factors like pressure, temperature, the concentration of reactants.
Chemical Reaction
Chemical reactions are chemical changes in which reactants transform into products by making or breaking bonds (or both) between different atoms.
A chemical reaction is a process that causes one set of chemical components to change into another. Chemical reactions are defined as changes in the locations of electrons in the formation and breaking of chemical bonds between atoms, with no change in the nuclei, and are described using a chemical equation. At a given temperature and chemical concentration, chemical reactions occur at a predictable rate. Reaction speeds often increase as the temperature rises because more thermal energy is available to attain the activation energy required to break bonds between atoms.
Types of Chemical Reactions
The basis for different types of reactions is the product formed, the changes that occur, the reactants involved and so on. Different types of reactions are
- Endothermic
- Exothermic
- Combustion reaction
- Decomposition reaction
- Neutralization reaction
- Redox Reaction
- Single Displacement
- Precipitation or Double-Displacement Reaction
- Synthesis reaction
1. Combustion Reaction
A combustion reaction is a reaction with a combustible material with an oxidizer to give an oxidized product. An oxidizer is a chemical a fuel requires to burn, generally oxygen. Consider the example of combustion of magnesium metal.
Here, 2 magnesium atoms react with a molecule of oxygen producing 2 molecules of the compound magnesium oxide releasing some heat in the process.
2. Decomposition Reaction
A Decomposition reaction is a reaction in which a single component breaks down into multiple products. Certain changes in energy in the environment have to be made like heat, light or electricity breaking bonds of the compound. Consider the example of the decomposition of calcium carbonate giving out CaO (Quick Lime) which is a major component of cement.
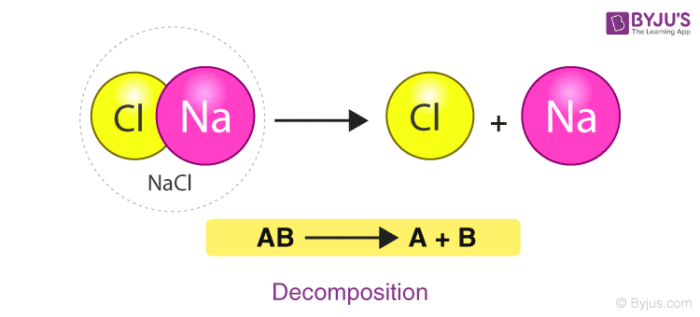
Here, the compound Calcium carbonate when heated breaks down into Calcium Oxide and Carbon Dioxide.
3. Neutralization Reaction
A Neutralization reaction is basically the reaction between an acid and a base giving salt and water as the products. The water molecule formed is by the combination of OH– ions and H+ ions. The overall pH of the products when a strong acid and a strong base undergo a neutralization reaction will be 7. Consider the example of the neutralization reaction between Hydrochloric acid and Sodium Hydroxide giving out sodium chloride(Common Salt) and water.
Here, an acid and a base, Hydrochloric acid and Sodium Hydroxide react in a neutralization reaction to produce Sodium Chloride(Common Salt) and water as the products.
4. Redox Reaction
A REDuction-OXidation reaction is a reaction in which there is a transfer of electrons between chemical species. Let us consider the example of an electrochemical cell-like redox reaction between Zinc and Hydrogen.
Here, A Zinc atom reacts with 2 ions of positively charged hydrogen to which electrons get transferred from the zinc atom and hydrogen becomes a stable molecule and Zinc ion is the product.
5. Precipitation or Double-Displacement Reaction
It is a type of displacement reaction in which two compounds react and consequently, their anions and cations switch places forming two new products. Consider the example of the reaction between silver nitrate and sodium chloride. The products will be silver chloride and sodium nitrate after the double-displacement reaction.
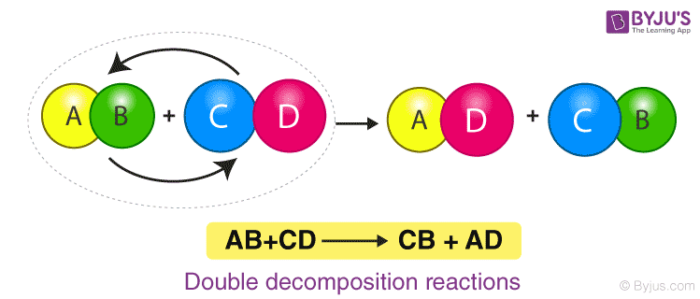
Here, Silver Nitrate and Sodium Chloride undergo a double displacement reaction. Wherein Silver replaces Sodium in Sodium Chloride and Sodium joins with Nitrate becoming Sodium Nitrate along with the Silver Chloride as the product.
6. Synthesis Reaction
A Synthesis reaction is one of the most basic types of reaction wherein multiple simple compounds combine under certain physical conditions giving out a complex product. The product will always be a compound. Let us consider the Synthesis reaction of sodium chloride with reactants solid sodium and chloride gas.
Here, we have 2 Atoms of solid Sodium reacting with Chlorine gas giving out Sodium Chloride viz. Common Salt as the product.
Important Points to Remember
- In a chemical change, a new compound is formed but in a physical change, the substance changes its state of existence.
- Atoms or ions or molecules which react to form a new substance are called reactants; the new atoms or molecules formed are products.
- A chemical reaction follows the law of conservation of mass. That is no atom is destroyed or created but only a new product is formed from reactants.
Chemical Reactions
A chemical reaction occurs when one or more reactants are changed into one or more products. The constituent atoms of the reactants are rearranged in a chemical reaction, resulting in the formation of various substances as products.
Physical and Chemical Changes
Chemical change – one or more new substances with new physical and chemical properties are formed.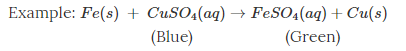
Here, when copper sulphate reacts with iron, two new substances, i.e., ferrous sulphate and copper, are formed.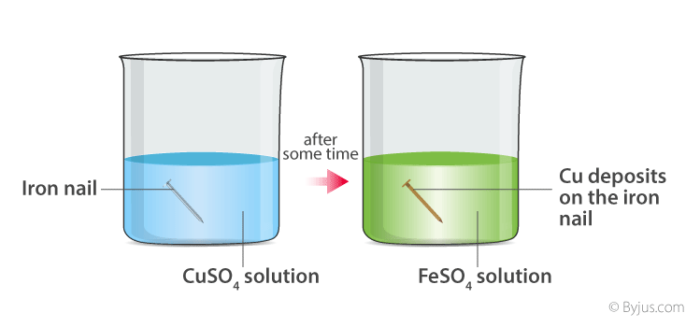
Physical change – change in colour or state occurs, but no new substance is formed.
Example: Water changes to steam on boiling, but no new substance is formed (Even though steam and water look different when they are made to react with a piece of Na, they react the same way and give the exact same products). This involves only a change in state (liquid to vapour).
Observations that Help Determine a Chemical Reaction
A chemical reaction can be determined with the help of any of the following observations.
a) Evolution of a gas
b) Change in temperature
c) Formation of a precipitate
d) Change in colour
e) Change of state
Chemical Equations
Due to the vast amounts of chemical reactions happening around us, a nomenclature was developed to simplify how we express a chemical reaction in the form of a chemical equation. A chemical equation is nothing but a mathematical statement which symbolizes the product formation from reactants while stating certain condition for which how the reaction has been conducted.
The reactants are on the left-hand side whereas the products formed are on the right-hand side. The reactants and products are connected by a one-headed or two-headed arrows. For example, a reaction
A + B → C + D
Here, A and B are the reactants, which react to form the products C and D. In an actual chemical equation, reactants are denoted by their chemical formula. In order to assure the law of conservation of mass, a chemical equation must be balanced i.e. the number of atoms on both sides must be equal. This is the balancing of the equation.
Let us consider an actual chemical reaction between Methane(CH₄) and Oxygen (O2),
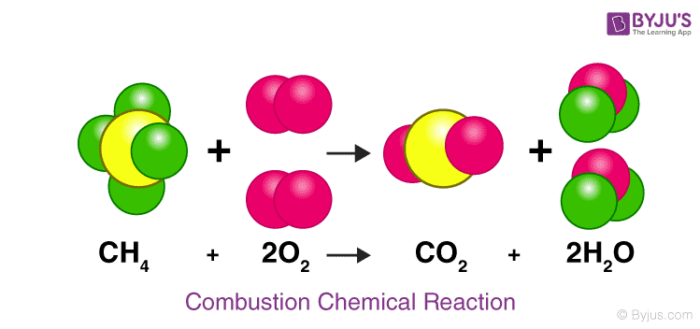
Here we can see how the number of each atom on the left side is balanced on the right side, as stated by the law of conservation of mass.
Chemical Reactions and Equations I
Word Equation
A word equation is a chemical reaction expressed in words rather than chemical formulas. It helps identify the reactants and products in a chemical reaction.
A chemical reaction is described using a word equation, which is a shorthand manner of expressing it. The names of the reactants are shown on the left side of a word equation. If there is more than one reactant, the names of the reactants are separated by a plus sign (+). Products are shown on the right side of a word equation. If there is more than one product, the names of the products are separated by a plus sign (+).
For example,
Sodium + Chlorine → Sodium chloride
The above equation means: “Sodium reacts with chlorine to form sodium chloride.”
Symbols of Elements and Their Valencies
A symbol is a chemical code for an element. Each element has a one or two-letter atomic symbol, which is, in most cases, the abbreviated form of its name.
Valency is the combining capacity of an element. It can be considered as the number of electrons lost, gained or shared by an atom when it combines with another atom to form a molecule.
Writing Chemical Equations
Representation of a chemical reaction in terms of symbols and chemical formulae of the reactants and products is known as a chemical equation.
![]()
• For solids, the symbol is “(s)”.
• For liquids, it is “(l)”.
• For gases, it is “(g)”.
• For aqueous solutions, it is “(aq)”.
• For gas produced in the reaction, it is represented by “(↑)”.
• For precipitate formed in the reaction, it is represented by “(↓)”.
Balancing of a Chemical Reaction
Law of Conservation of Mass
According to the Law of Conservation of Mass, no atoms can be created or destroyed in a chemical reaction, so the number of atoms for each element on the reactants side has to balance the number of atoms that are present on the products side.
In other words, the total mass of the products formed in a chemical reaction is equal to the total mass of the reactants participating in a chemical reaction.
Balanced chemical equation
The chemical equation in which the number of atoms of each element on the reactants side is equal to that of the products side is called a balanced chemical equation.
Steps for Balancing Chemical Equations
The changes that occur during a chemical reaction are represented by a chemical equation.
Reactants → Products
The equilibrium of all chemical equations must be maintained. This means that on both sides of the arrow, the number of each sort of atom must be the same.
Chemical equations are balanced using coefficients. A coefficient is a numerical value that is added to the front of a chemical symbol or formula. It indicates the number of atoms or molecules of the material involved in the process.
Place coefficients in front of symbols or formulas as needed to balance a chemical equation so that the same number of each type of atom appears in both reactants and products.
For example,
Zn + HCl → ZnCl2 + H2
The balanced equation is
Zn + 2HCl → ZnCl2 + H2
Hit and trial method: While balancing the equation, change the coefficients (the numbers in front of the compound or molecule) so that the number of atoms of each element is the same on each side of the chemical equation.
Short-Cut Technique for Balancing a Chemical Equation
Example:
aCaCO3 + bH3PO4 → cCa3(PO4)2 + dH2CO3
Set up a series of simultaneous equations, one for each element.
Ca: a=3c
C: a=d
O: 3a+4b=8c+3d
H: 3b=2d
P: b=2c
Let’s set c=1
Then a=3 and
d = a = 3
b = 2c = 2
So a=3; b=2; c=1; d=3
The balanced equation is
3CaCO3 + 2H3PO4 → Ca3 (PO4)2 + 3H2CO3
Chemical Reactions and Equations II
Types of Chemical Reactions
Taking into consideration different factors, chemical reactions are grouped into multiple categories.
A few examples are:
● Combination
● Decomposition
● Single Displacement
● Double displacement
● Redox
● Endothermic
● Exothermic
● Precipitation
● Neutralisation
Combination Reaction
In a combination reaction, two elements or one element and one compound or two compounds combine to give one single product.
When quicklime or calcium oxide (CaO) reacts with water, slaked lime [Ca(OH)2] is formed. During this reaction, a large amount of heat is released. So, this reaction is an exothermic Reaction.
CaO + H2O → Ca(OH)2
Decomposition Reaction
A single reactant decomposes on the application of heat or light, or electricity to give two or more products.
Types of decomposition reactions:
a. Decomposition reactions which require heat-thermolytic decomposition or thermolysis.

b. Decomposition reactions which require light-photolytic decomposition or photolysis.

c. Decomposition reactions which require electricity – electrolytic decomposition or electrolysis.
Displacement Reaction
A more reactive element displaces a less reactive element from its compound or solution.

Double Displacement Reaction or Precipitation Reaction
An exchange of ions between the reactants takes place to give new products.
For example, ![]()
An insoluble compound called precipitate forms when two solutions containing soluble salts are combined.![]()
One of the best examples of precipitation reactions is the chemical reaction between potassium chloride and silver nitrate, in which solid silver chloride is precipitated out. This is the insoluble salt formed as a product of the precipitation reaction. The chemical equation for this precipitation reaction is provided below.
AgNO3(aqueous) + KCl(aqueous) —–AgCl(precipitate) + KNO3(aqueous)
Redox Reaction
A redox reaction occurs when the oxidation states of the substrate change. The loss of electrons or an increase in the oxidation state of a chemical or its atoms is referred to as oxidation. The gain of electrons or a decrease in the oxidation state of a chemical or its atoms is referred to as reduction.
Oxidation and reduction take place simultaneously.
Oxidation: Substance loses electrons or gains oxygen or loses hydrogen.
Reduction: Substance gains electrons or loses oxygen or gains hydrogen.
Oxidising agent – a substance that oxidises another substance and self-gets reduced.
Reducing agent – a substance that reduces another substance and self-gets oxidised.

Endothermic and Exothermic reaction
Exothermic reaction – heat is evolved during a reaction. Most of the combination reactions are exothermic.
Al + Fe2O3 → Al2O3 + Fe + heat
CH4 + 2O2 → CO2 + 2H2O + heat
Effect of Oxidation Reaction in Everyday Life
Endothermic – Heat is required to carry out the reaction.
6CO2 + 6H2O + Sunlight → C6H12O6 + 6O2
Glucose
Most of the decomposition reactions are endothermic.
Corrosion
Gradual deterioration of a material, usually a metal, by the action of moisture, air or chemicals in the surrounding environment.
Rusting:
4Fe(s) + 3O2(from air) + xH2O(moisture) → 2Fe2O3.xH2O(rust)
Corrosion of copper:
Cu(s) + H2O(moisture) + CO2(from air) → CuCO3.Cu(OH)2(green)
Corrosion of silver:
Ag(s) + H2S (from air) → Ag2S(black) + H2(g)
Rancidity
It refers to the oxidation of fats and oils in food that is kept for a long time. It gives foul smell and bad taste to food. Rancid food causes stomach infections during consumption.
Prevention:
(i) Use of air-tight containers
(ii) Packaging with nitrogen
(iii) Refrigeration
(iv) Addition of antioxidants or preservatives








0 Comments